The Luminescence in Self Activated and Sm3+ rich Garnet Phosphor Sr2NaZn2V3O12 prepared by Solid-State Reaction
DOI:
https://doi.org/10.61343/jcm.v2i02.87Keywords:
Luminescence, garnet phosphor, vanadate garnet phosphor, Sr2NaZn2V3O12:Sm3+Abstract
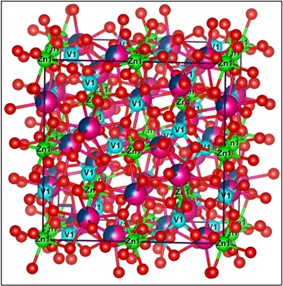
A new self-activated vanadate garnet phosphor Sr2NaZn2V3O12 and doped with Sm3+ vanadate garnet phosphor Sr2NaZn2V3O12 were produced by a simple conventional solid-state reaction that calcined for six hours at 950 degrees Celsius and then left to cool to room temperature. Vanadate garnet phosphor is utilized for high-performance tasks because of its excellent strength and hardness. The single phase garnet structures in the prepared materials have been verified by X-ray diffraction (XRD) and Rietveld polishing. The behaviors of rare earth-doped luminescence and self-activated luminescence have been thoroughly examined. The prepared Sr2NaZn2V3O12 compound was observed a wide-band at wavelength of 485 in visible region of greenish emission that originates due to VO43− emission. With lifetime measurement and photoluminescence (PL), In Sr2NaZn2V3O12: Sm3+ garnet-type phosphors, the energy transfer characteristics from VO43− (vanadate) to Sm3+ (samarium trivalent) ions have been shown. While excitation was reported at 485 nm for vanadate garnet phosphor and 601 nm for samarium-rich garnet phosphor, the produced materials were excited by 338 nm for vanadate and 405 nm for Sm3+ rich phosphor. Scanning electron microscopy was used to study the topography and morphology. The findings suggest that Sm3+ doped Sr2NaZn2V3O12 garnet phosphors and self-activated Sr2NaZn2V3O12 exhibit significant promise for use in near-UV stimulated white LEDs.
References
C. Wang, J. Zhou, L. Lulu and Q. Song, Part. Part. Syst. Charact., 35: 1700387, 2018.
S. van Herwaarden, Sens. Mater., 8: 373, 1996.
C. A. Geiger, the laboratory and technology Elements, 9 (6): 447–452, 2013.
J. Ueda and S. Tanabe, Human and Environmental Studies, Kyoto, 606- 8501, 2019.
A. Markovskyi, V. Gorbenko, T. Zorenko, T. Yokosawa, J. Will, E. Spiecker, M. Batentschuk, J. Elia, A. Fedorov and Y. Zorenko, Cryst. Eng. Comm. 23, 2021.
P. Du, Y. Hua and J. S. Yu, Chem. Eng. J., 352: 352, 2018.
H. Xie, T. Tsuboi, W. Huang, Y. Huang, L. Qin and H. J. Seo, J. Am. Ceram. Soc., 97:1434, 2018.
A. Bindhu, J. I. Naseemabeevi and S. Ganesanpotti, Crit. Rev. Solid State Mater. Sci., 47: 621, 2021.
A. Bindhu, A. S. Priya, J. I. Naseemabeevi and S. Ganesanpotti, J. Alloys Compd., 893, 162246, 2022.
P. P. Lohe, D. V. Nandanwar, P. D. Belsare, and S. V. Moharil, AIP Conference Proceedings, 2104: 020015, 2019.
J. Li, B. Liu, G. Liu, Q. Che, Y. Lu and Z. Liu, J. Rare Earths, 2022.
S. Kaur, A.S. Rao and M. Jayasimhadri, J. Lumin. 202: 461-468, 2018.
M. D. Birowosuto, M. Isnaeni, C. de Mello Doneg_a, and A. Meijerink, J. Appl. Phys. 118: 123105, 2015.
Downloads
Published
How to Cite
Issue
Section
Categories
License
Copyright (c) 2025 Ganesh Vandile , D. V. Nandanwar, A. K. Nandanwar, D. W. Akhare

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright© by the author(s). Published by journal of Condensed Matter. This is an open access article distributed under the terms of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.









