Photo-physical properties of Pr (III) chelates of substituted nitrobenzoic acid and nitrophenols
DOI:
https://doi.org/10.61343/jcm.v1i01.7Keywords:
Praseodymium, Substituted nitrobenzoic acid, Substituted nitrophenols, Photo physical properties, Judd Ofelt parameter, Laser parameterAbstract
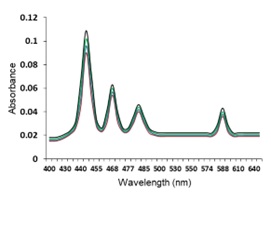
Electronic absorption and emission spectra were recorded for chelates of Pr (III) with 2-hydroxy-4-nirobenzoic acid, 3-hydroxy-4-nitrobenzoic acid, 4-hydroxy-3-nitrobenzoic acid, 4-methyl-2-nitrophenol, 4-chloro-2-nitrophenol and 5-fluoro-2-nitrophenol in various M: L stoichiometry and for different pH. Intensity and energy of intraconfigurational 4fn transitions have been determined from the absorption spectra. The spectroscopic parameters like Slater-Condon (Fk), Racah (Ek), Lande (ζ4f) and Judd-Oflet parameters Ωλ (λ=2, 4, 6) have been computed using statistical method like partial regression method. The Judd-Oflet intensity parameters and fluorescence spectra have been used to calculate radiative life time (τ) of two excited states 3P0 and 1D2. From the fluorescence spectra of the chelates, effective line width (Δλeff) spontaneous emission probability (A), fluorescence branching ratio (β) and stimulated emission cross section (σ) have been determined for three optical transition 3P0-3H4, 3P0-3H5 and 1D2-3H4. Spectroscopic and intensity parameters were studied with respect to the ligand field symmetry and degree of bond covalency.
References
C. H. Huang: “Rare Earth Coordination Chemistry Fundamentals and Applications”;
I edition, John Wiley & Sons, 2010.
S. Laurent, L. Vander, R. N. Muller: “Lanthanide complexes for magnetic resonance and optical molecular imaging”; Quaterly Journal of Nuclear Medicine and Molecular Imaging, 53 (6), 2009, pp-586-603.
E. G. Moore, A. P. Samuel, K. N. Raymond: “From antenna to assay: lessons learned in lanthanide luminescence”; Accounts of Chemical Research, 42(4), 2009, pp-542-52.
V. M. Runge: “Advances in magnetic resonance”; Investigative Radiology, 43 (12), 2008, pp-893-898.
M. A. Katkova, M. N. Bochkarev: “New trends in design of electroluminescent rare earth metallo-complexes for OLEDs”; Dalton Transactions, 39, 2010, pp-6599-6612.
L. Li, C. Tsung, Z. Yang, et al.: “Rare-earth-doped nanocrystalline titania microspheres emitting luminescence via energy transfer”; Advanced Materials, 20 (5), 2008, pp. 903–908.
H. Song, G. Pan, X. Bai, S. Li, H. Yu, and H. Zhang: “One-dimensional rare earth compounds and complexes: preparation and improved photoluminescence properties”; Journal of Nanoscience and Nanotechnology, 8 (3), 2008, pp-1316–1325.
S. N. Misra, M. A. Gagnani, Indira Devi M., and R. S. Shukla: “Biological and Clinical Aspects of Lanthanide Coordination Compounds”; Bioinorganic Chemistry and Applications, 2(3-4), 2004, pp-155–192.
J. Comley: “TR-FRET Based Assays-Getting Better with Age. Drug Discovery World”; spring 2006, pp-22–37.
R. P. Mathur, K. G. Sharma & R. K. Mehta; Proc. Indian Natn. Sci. Acad., 47 A, (1981), 322.
R. P. Mathur, K. G. Sharma & R. K. Mehta; J. Indian Chem. Soc., 58, (1981), 225.
R. P. Mathur, C. P. Gupta, Vibha Suri & R. K. Mehta; Proc. Indian Natn Sci. Acad., 48 A, (1982), 232.
R. P. Mathur, K. C. Mehta & R. K. Mehta; Trans. SAEST, 17, (1982), 39.
R. P. Mathur, C. P. Gupta, K. G. Sharma & R. K. Mehta; Acta. Chim. Acad. Sci. Hungaricae, 111, (1982), 19.
R. P. Mathur, K. G. Sharma & R. K. Mehta; Egypt. J. Chem., 25, (1982), 409.
R. P. Mathur, Praveen Mathur & R. K. Mehta, J. Indian Chem. Soc., 60, (1983), 107.
R. P. Mathur, Veena Praadhan & S.P. Mathur; Trans. SAEST, 25, (1990), 9.
R. P. Mathur, Veena Pradhan & Praveen Mathur; Chemica Acta., Turcia (Turkey), 22, (1994), 57.
R. P. Mathur and Anu Sharma; Asian J. Chem., 12, (2000), 615.
R. P. Mathur and R. S Verma; Oriental J. Chem., 18, (2002), 73.
Alok Vyas and R.P. Mathur; “pH metric and thermodynamic studies of rare earth chelates”, Journal of Rare earths, 26, (2008), 85-88.
W. Ference, Beata Bocian and Daria Mazur: “Spectral and thermal properties of light lanthanide complexes with 4-methoxy-3-nitrobenzoic acid”; Crotica Chemica Acta, 72 (4), 1999, pp779-787.
Sock-Sung Yun, Hong-Ryol Suh et.al: “Lanthanide complexes of some high energetic compounds (III), crystal structures and thermal properties of 2,6-dinitrophenol(2,6-DNP) complexes”; Journal of Alloys and Compounds, Elsevier, Vol 408-412, pp1030-1036.
De Bettencourt-Dias A, Viswanathan S., “Nitrofunctionalisation and luminescence of quantum yield of Eu(III) and Tb(III) benzoic acid complexes”; Dalton Trans, 2006, September 14(34);4093-103.
Tatjana N.Parac-Vogt, Sophia Pachini et.al; “Lanthanide (III) Nitrobenzene sulfonates as New Nitration Catalyst: The Role of the Metal and of the Counter ion in the Catalytic Efficiency”; European Journal of Organic Chemistry, 2004, pp 4560-4566.
B. R. Judd; “Optical absorption intensities of rare earth ions”; Physics Review, 127, (1962), 750.
G. S. Oflet; “Intensities of crystal spectra of rare earth ions”; Journal of Chemical Physics, 37, (1962), 511.
J. C. Slater; Phys. Rev, 34, (1929), 1293.
E. U. Condon & G. H. Shortely; “The Theory of Atomic Spectra”, (1963), University Press, Cambridge.
G. Racah; Phys. Rev. 61, (1942), 186.
G. Racah; Phys. Rev. 62, (1942), 438.
G. Racah; Phys. Rev. 63, (1942), 367.
W.T. Carnell, P. R. Fields & B. G. Wybourne; J. Chem Phys., 42(11), (1965), 3797.
E.Y. Wong, J. Chem. Phys., 35, (1961), 544.
E.Y. Wong, J. Chem. Phys., 38, (1963), 976.
B. G. Wybourne; “Spectroscopic Properties of the Rare Earths”, (1965), Interscience, New York.
D. E. Henrie and G. R. Choppin; J. Chem. Phys., 49, (1968), 477
W. T. Carnell, P. R. Fields & K. Rajnak; “Electronic Energy levels in the trivalent lanthanide aqua ions I Pr3+, Nd 3+, Pm3+, Sm3+, Dy3+, Ho3+, Er3+, Tm3+, Journal of Chemical Physics, 49, (1968), 4412, 4424, 4443.
B. Bhatia, M.P. Bhutra and S.P. Tandon; “Spectroscopic study of Pr3+ amino acid ternary complexes in aqueous solution; Revision Tec. Ing. Univ. Zulia, 11, (1988), 69-72.
C. K. Jorgensen, R. Reisfield; Journal of less common Metals, 93, (1983), 107.
K. Driesen, C. G. Warland, K. Binnemans; “Spectroscopic properties of Sol-Gel glasses doped with lanthanide bipyridyl complexes”; Material Science And Engineering C 18, (2001), 25-258.
W. F. Krupke; IEEE. J. Quantum Electron, QE-7, (1971), 153.
W. F. Krupke; IEEE. J. Quantum Electron, QE-10, (1974), 450.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Vyas A, Vyas M, Manoj S Shekhawat

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright© by the author(s). Published by journal of Condensed Matter. This is an open access article distributed under the terms of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.









