Understanding the behavior of 5, 10, 15, 20-tetrakis (4 -hydroxyphenyl) porphyrin and its cation in Methanol: insights from electronic structure calculations
DOI:
https://doi.org/10.61343/jcm.v1i02.27Keywords:
Solvation, porphyrin, DFT, energy gap, charge transferAbstract
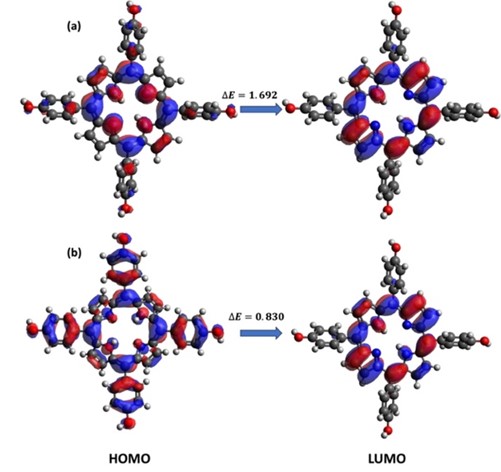
This research investigates the solvation dynamics and interactions of neutral 5,10,15,20-tetrakis(4-hydroxyphenyl) porphyrin (TPPH) and its cationic form (TPPH2+) with methanol as the solvent. HOMO-LUMO analysis and Global Chemical Reactive Descriptors (GCRD) results were reported using DFT method with BP86 functional. The study reveals contrasting charge transfer behaviors: neutral TPPH demonstrates an enhanced charge transfer rate upon dissolution in methanol, while cationic TPPH exhibits a reverse trend. This solvation-induced reduction in energy gap presents a potential avenue for optimizing optoelectronic devices like light-emitting diodes and laser diodes. These findings elucidate the intricate interplay between porphyrin derivatives and solvents, offering valuable insights for tailored applications across diverse scientific and technological fields.
References
M.V. Tesakova and V.I. Parfenyuk, Surf. Engin. Appl. Electrochem. 57, 67, 2021.
Ioana Baldea, et. al, Processes, 11, 917, 2023.
Rica Boscencu et.al, Molecules, 28(3):1149, 2023.
F. Neese, et. al, J. Chem. Phys. 152, 22, 224108, 2020.
Louise M. Debefve and Christopher J. Pollock, Phys. Chem. Chem. Phys. 23, 25780, 2021.
M. Mohsen-Nia, H. Amiri and B. Jazi, Journal of Solution Chemistry, 39, 701, 2010.
Takao Tsuneda, et. al, J. Chem. Phys. 133, 174101, 2010.
P.K. Chattaraj, et. al, Chem. Rev. 107, 9, PR46, 2007.
Marzieh Miar, et. al, Journal of Chemical Research, 45, 147, 2021.
A.G. Pramod, et. al, Journal of Molecular Liquids, 292, 111383, 2019.
Downloads
Published
How to Cite
License
Copyright (c) 2023 Anju

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright© by the author(s). Published by journal of Condensed Matter. This is an open access article distributed under the terms of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.









