Oxidation Kinetics Studies of Ti3C2Tx MXene using Freeman-Carroll method
DOI:
https://doi.org/10.61343/jcm.v2i02.108Keywords:
Ti3C2Tx MXene, Kinetics, Oxidation activation energy, Freeman Carrol methodAbstract
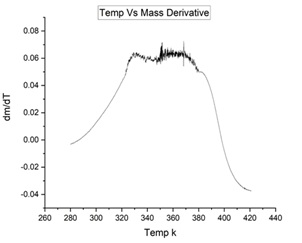
Ti3C2Tx MXene is synthesized from Ti3Al2C3 MAX phase by using HF treatment and characterized by X-ray diffraction In addition, oxidation of Ti3C2Tx MXene in nitrogen environments from room temperature to 500 oC is studied by thermogravimetric analysis. The experiment shows that oxidation of Ti3C2Tx MXene starts at 275 oC. Activation energy of oxidation of Ti3C2Tx MXene is determined using the Freeman Carroll method. It is found that in a nitrogen environment and in a strong oxidation temperature range, oxidation activation energy of Ti3C2Tx MXene is approximately 235.6 kJ mol-1.
References
S. Kumar, M. A. Rehman, S. Lee, M. Kim, H. Hong, J. Y. Park & Y. Seo Sci. Rep. 11, 649- 2021.
A.M. Iquba, A. Tariq A. Zaheer S. Gul, A.AI AliM, Z. Iqbal D. Akinwandeand S. Rizwan ASC Omega, 4(24), 20530-20539, 2019.
Y. Wang and Y. Wang, Smartmat 4(1), 2-25, 2022
B. Shen, R. Hao, Y. Huang, Z. Guo and X. Zhu, Crystals 12, 1099, 2-38, 2022.
D. Yang, H Sun, H. Lu, Y. Guo X. Li and X. Hu, Supercond. Sci. Technol. 16;576–581, 2003.
Downloads
Published
How to Cite
License
Copyright (c) 2025 Anarse D A, Kadam M B, Sunatkari A L, Chavan A U, Prasad M R, Sarawade P B

This work is licensed under a Creative Commons Attribution 4.0 International License.
Copyright© by the author(s). Published by journal of Condensed Matter. This is an open access article distributed under the terms of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.









