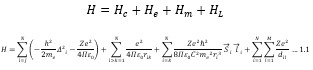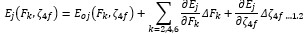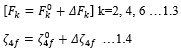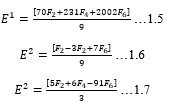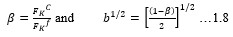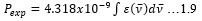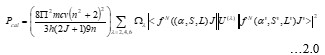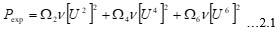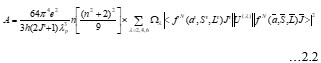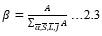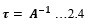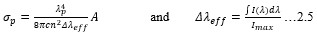Photo-physical properties of Pr (III) chelates of substituted nitrobenzoic acid and nitrophenols
Vyas A1*, Vyas M2, Shekhawat M3
DOI:10.61343/jcm.v1i01.7
1* Alok Vyas, Department Of Science And Humanities, GPC, Bikaner, Raj, India.
2 Mahendra Vyas, Department of Chemistry, Engineering College Bikaner, Bikaner, Raj, India.
3 Manoj S Shekhawat, Department of Physics, Engineering College Bikaner, Bikaner, Raj, India.
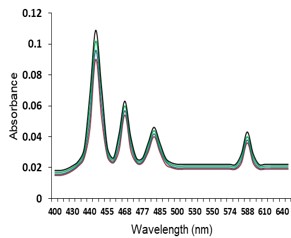
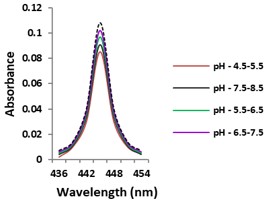
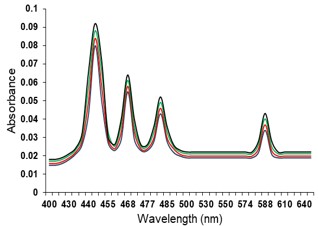
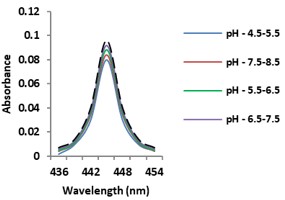
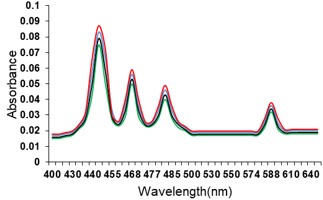
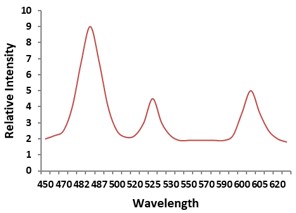
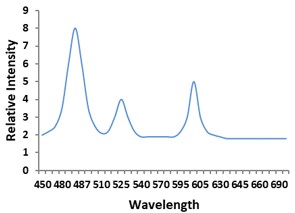
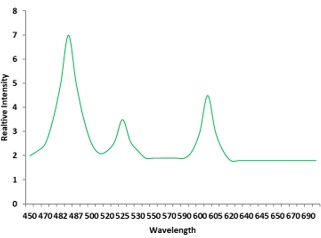
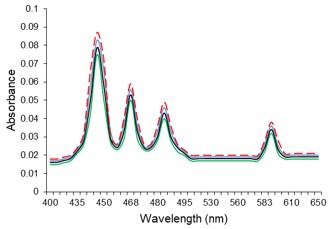
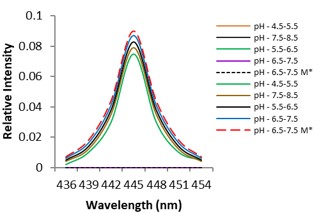
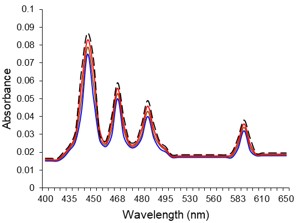
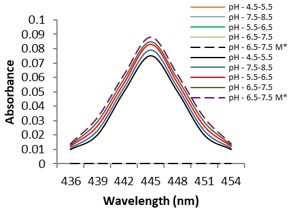
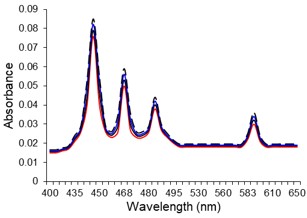
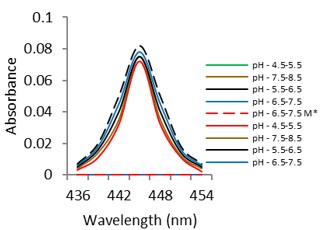
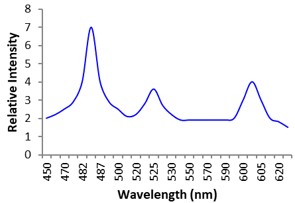
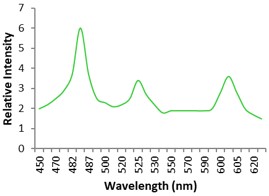
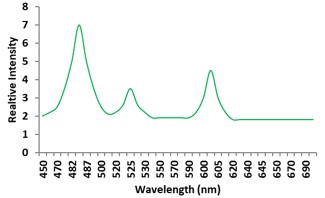
Electronic absorption and emission spectra were recorded for chelates of Pr (III) with 2-hydroxy-4-nirobenzoic acid, 3-hydroxy-4-nitrobenzoic acid, 4-hydroxy-3-nitrobenzoic acid, 4-methyl-2-nitrophenol, 4-chloro-2-nitrophenol and 5-fluoro-2-nitrophenol in various M: L stoichiometry and for different pH. Intensity and energy of intraconfigurational 4fn transitions have been determined from the absorption spectra. The spectroscopic parameters like Slater-Condon (Fk), Racah (Ek), Lande (ζ4f) and Judd-Oflet parameters Ωλ (λ=2, 4, 6) have been computed using statistical method like partial regression method. The Judd-Oflet intensity parameters and fluorescence spectra have been used to calculate radiative life time (τ) of two excited states 3P0 and 1D2. From the fluorescence spectra of the chelates, effective line width (Δλeff) spontaneous emission probability (A), fluorescence branching ratio (β) and stimulated emission cross section (σ) have been determined for three optical transition 3P0-3H4, 3P0-3H5 and 1D2-3H4. Spectroscopic and intensity parameters were studied with respect to the ligand field symmetry and degree of bond covalency.
Keywords: Praseodymium, Substituted nitrobenzoic acid, Substituted nitrophenols, Photo physical properties, Judd Ofelt parameter, Laser parameter
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , , Department Of Science And Humanities, GPC, Bikaner, Raj, India. Email: |
Vyas A, Vyas M, Shekhawat M, Photo-physical properties of Pr (III) chelates of substituted nitrobenzoic acid and nitrophenols. J.Con.Ma. 2023;1(1):34-45. Available From https://jcm.thecmrs.in/index.php/j/article/view/7 |


 ©
©