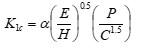Optical and Mechanical Properties of Lithium Aluminosilicate Glass-ceramics
Kumar A1*, Chakrabarti A2, Chatterjee S3, Shekhawat M4, Molla A5
DOI:10.61343/jcm.v1i01.5
1* Anil Kumar, Department Of Physics, Seth G L Bihani S D Pg College, Sri Ganganagar 335001, Rajasthan, India.
2 Anirban Chakrabarti, Department of SolidState Physics , Indian Association for the Cultivation of Science, Kolkata 700032, West Bengal, India.
3 Shaona Chatterjee, Specialty Glass Division, CSIR Central Glass and Ceramic Research Institute, Kolkata700032, West Bengal, India.
4 Manoj S Shekhawat, Department of Physics, Engineering College Bikaner, Bikaner 334004, Rajasthan, India.
5 Atiar Rahaman Molla, Specialty Glass Division, CSIR Central Glass and Ceramic Research Institute, Kolkata700032, West Bengal, India.
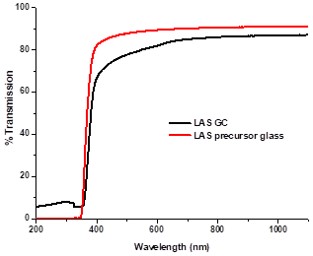
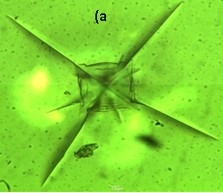
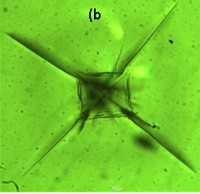
These glasses prepared by melt-quench technique. High transparency (>80%) in the visible wavelength range has been observed in the material by UV-VIS-NIR spectrometry. FTIR spectra indicated the presence of Si-O as well as Al-O bonds in the glass-ceramics. The LAS glass ceramics showed moderate flexural strength (~80 MPa) and young’s modulus (~50 GPa). Microstructural characterization of the heat-treated glass ceramics by FESEM and TEM showed nano-crystalline spherical particles of 30-40 nm, which provided a rationale for its high transparency and good mechanical properties that may open up possibilities for newer applications.
Keywords: Lithium Aluminosilicate glasses, TEM, FESEM
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , , Department Of Physics, Seth G L Bihani S D Pg College, Sri Ganganagar 335001, Rajasthan, India. Email: |
Kumar A, Chakrabarti A, Chatterjee S, Shekhawat M, Molla A, Optical and Mechanical Properties of Lithium Aluminosilicate Glass-ceramics. J.Con.Ma. 2023;1(1):24-28. Available From https://jcm.thecmrs.in/index.php/j/article/view/5 |


 ©
©