Sol-Gel Synthesis, Crystalline Size, Dislocation Density and Microstrain of LiNi0.85Co0.10Mn0.05O2 Cathode Material for Lithium-ion Batteries
Monika1, Mishrab A2, Patial B3*
DOI:10.61343/jcm.v1i02.46
1 Monika, Department Of Physics, Himachal Pradesh University Summerhill, Shimla, Himachal Pradesh 171005, India.
2 Ashish Kumar Mishrab, Department Of Physics, Himachal Pradesh University, Summerhill Shimla, Himachal Pradesh 171005, India.
3* Balbir Singh Patial, Department Of Physics, Himachal Pradesh University, Summerhill Shimla, Himachal Pradesh171005, India.
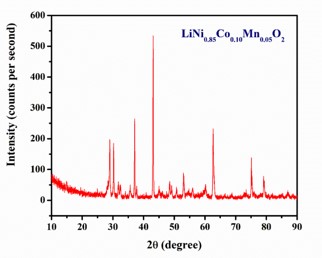
Lithium-ion batteries (LIBs) are essential energy storage solutions for a wide range of applications. The cathode material significantly influences the performance of LIBs. Nickel-cobalt-manganese (NCM) ternary cathode materials have gained prominence due to their potential to offer high capacity, stability and voltage characteristics. In this paper, we focus on the synthesis of NCM cathode material using sol-gel method and its characterization primarily through X-ray diffraction (XRD) analysis. The crystal structure of the synthesized material is investigated using XRD. These XRD patterns are analyzed to estimate particle size and to deduce crystalline size, dislocation density and microstrain. This study helps us better understand how NCM materials are put together, which is important for making high performance lithium-ion batteries. These batteries are used in laptops, electric cars, etc.
Keywords: Lithium-ion batteries, cathode material, NCM ternary cathode, sol-gel synthesis, X-ray diffraction.
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Department Of Physics, Himachal Pradesh University, Summerhill Shimla, Himachal Pradesh171005, India. Email: |
Monika, Mishrab A, Patial B, Sol-Gel Synthesis, Crystalline Size, Dislocation Density and Microstrain of LiNi0.85Co0.10Mn0.05O2 Cathode Material for Lithium-ion Batteries. J.Con.Ma. 2023;1(2):138-141. Available From https://jcm.thecmrs.in/index.php/j/article/view/46 |


 ©
© 
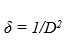 (1)
(1)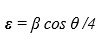 (2)
(2)