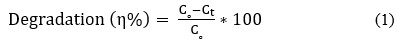Structural, Electric transport and Photocatalytic properties of Gd3+ / ZnO nanostructures
Chauhan S1, Gahlawat J2*, Sihag K3, Kumar P4
DOI:10.61343/jcm.v1i02.37
1 Shakshi Chauhan, Department of Physics, Baba Mastnath University, Rohtak, Haryana, India.
2* Jyoti Gahlawat, Department Of Physics, Ch Bansi Lal Government College For Women, Tosham, Haryana, India.
3 Kaushlya Sihag, Department of Physics, School Education, Panchkula, Haryana, India.
4 Praveen Kumar, Department of Physics, Ch Bansi Lal Government College for Women, Tosham, Haryana, India.
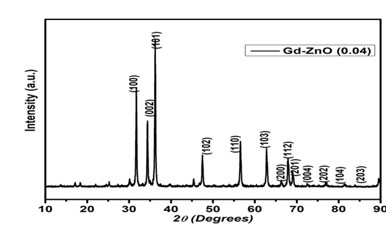
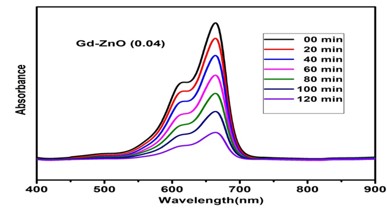
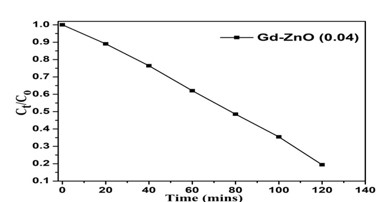
Metal oxide nanoparticles have gained a lot of popularity in recent years because of their broad band gap and several practical uses in solar, catalysis, sensors, actuators, and other domains. This work investigates the effects of Gd ion substitution on structural and electrical transportation properties in co-precipitated ZnO nanostructures. The prepared samples were characterized using XRD, field emission scanning electron microscopy, FT-IR (infrared spectroscopy), PL (photoluminescence), and complex impedance spectroscopy. The XRD data with crystallite size of 29 nm revealed the wurtzite hexagonal structure in space group P63mc. The morphology micrograph shows where the samples have accumulated. For two hours, a photocatalytic reactor was used to measure the photocatalytic degradation of Gd-doped ZnO nanoparticles. Degradation efficiencies (ϳ%) for the ZnO sample doped with Gd are 81 percent.
Keywords: Zinc Oxide, Gd, Photo-luminescence, visible light, Photocatalytic.
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , , Department Of Physics, Ch Bansi Lal Government College For Women, Tosham, Haryana, India. Email: |
Chauhan S, Gahlawat J, Sihag K, Kumar P, Structural, Electric transport and Photocatalytic properties of Gd3+ / ZnO nanostructures. J.Con.Ma. 2023;1(2):160-162. Available From https://jcm.thecmrs.in/index.php/j/article/view/37 |


 ©
©