Effect of Organic Entities on the Performance of Potassium Dihydrogen Phosphate (KDP) Crystals
Bade S1, Rasal Y2, Shirsat M3, Hussaini S4*
DOI:10.61343/jcm.v1i02.35
1 Sujata B Bade, Crystal Growth Laboratory, Department of Physics, Milliya Arts Science and Management Science College, Beed 431122, Maharashtra, India.
2 Y B Rasal, Crystal Growth Laboratory, Department Of Physics, Milliya Arts Science And Management Science College, Beed 431122, Maharashtra, India.
3 MD Shirsat, Rusa, Dr Babasaheb Ambedkar Marathwada University, Aurangabad 431005, Maharashtra, India.
4* S S Hussaini, Crystal Growth Laboratory, Department Of Physics, Milliya Arts Science And Management Science College, Beed 431122, Maharashtra, India.
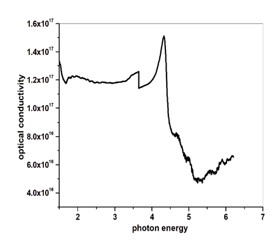
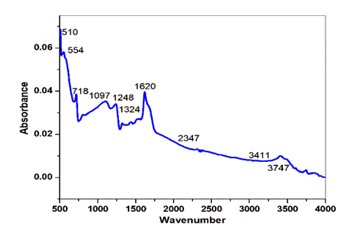
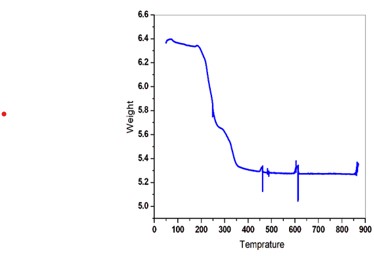
The 0.5 mol l-cystine and 2 mol oxalic acid doped in potassium dihydrogen phosphate crystals have been developed by slow evaporation solution technique at 20 °C. The powder X-ray diffraction technique was used to study the structural properties of the grown crystal. The FTIR spectral analysis method is used to identify the functional groups of grown crystals. The UV-visible was adopted to study the optical transparency of the grown crystals and their related optical parameters in the range of 200-900 nm. The optical transmittance is found to be 87 % of the grown crystal. The energy band gap (Eg) of the grown crystal observed from the graph is 3.93 eV. The Kurtz-Perry powder test is used to determine the SHG efficiency and it is observed that it is 0.5 times greater than pure KDP crystal.
Keywords: emerging trends, AR-grade potassium, Single-crystal X-ray
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Crystal Growth Laboratory, Department Of Physics, Milliya Arts Science And Management Science College, Beed 431122, Maharashtra, India. Email: |
Bade S, Rasal Y, Shirsat M, Hussaini S, Effect of Organic Entities on the Performance of Potassium Dihydrogen Phosphate (KDP) Crystals. J.Con.Ma. 2023;1(2):179-184. Available From https://jcm.thecmrs.in/index.php/j/article/view/32 |


 ©
©