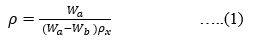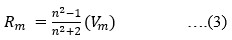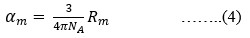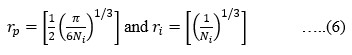Structural characterisation of Pr3+ ions incorporated sodium lead Barium borate glasses
Lenkennavar S1*
DOI:10.61343/jcm.v1i02.30
1* Susheela K Lenkennavar, Department Of Physics, Bangalore University, Bangalore 560056, Karnataka, India.
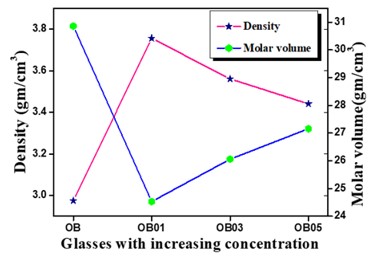
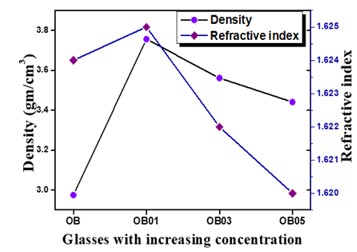
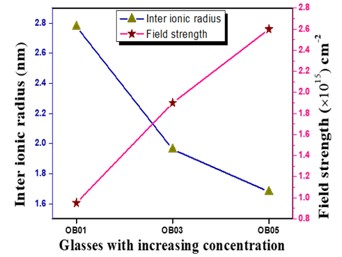
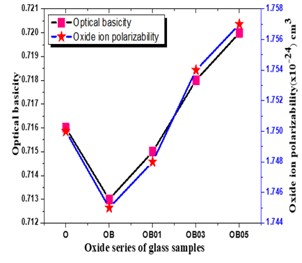
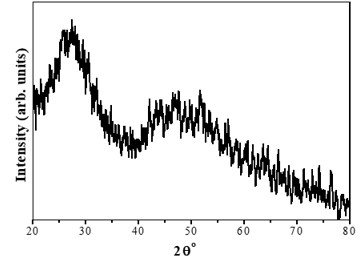
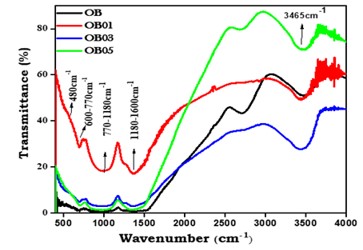
The Structural properties of 20Na2O – 10PbO – 10BaO –B2O3 – xPr2O3 glass doped with praseodymium have been investigated. The characterization techniques used for studying rare earth-doped glasses include energy-dispersive X-ray analysis (EDS), Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and scanning electron microscopy (SEM) for structural investigations. The concept of physical parameters plays a fundamental role in estimating the strength and structural compactness of the synthesized glass. Consequently, we investigated the influence of rare earth ions (Pr3+) concentration on physical properties. The nature and composition of the synthesized glass samples have been confirmed through Energy Dispersive X-ray Analysis (EDS) and Scanning Electron Microscopy (SEM). The results have been analyzed in view of the modified borate glass network.
Keywords: XRD, FTIR, SEM, EDS, optical basicity and numerical aperture
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , , Department Of Physics, Bangalore University, Bangalore 560056, Karnataka, India. Email: |
Lenkennavar S, Structural characterisation of Pr3+ ions incorporated sodium lead Barium borate glasses. J.Con.Ma. 2023;1(2):195-202. Available From https://jcm.thecmrs.in/index.php/j/article/view/30 |


 ©
©