A Comprehensive Examination of Ni@AgCl Nanoparticles – An Insight for Opto-Electronic Applications
Shivani RB1, R Vanathi Vijayalakshmi2*
DOI:10.61343/jcm.v1i02.29
1 Shivani RB, Department of Physics, Presidency College, Chennai- 600 005, Tamil Nadu, India.
2* R Vanathi Vijayalakshmi, Department Of Physics, Queen Marys College, Chennai600 005, Tamil Nadu, India.
Keywords: XRD, W-H Plot, UV-Vis absorption spectra, Photoluminescence..
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , , Department Of Physics, Queen Marys College, Chennai600 005, Tamil Nadu, India. Email: |
Shivani RB, R Vanathi Vijayalakshmi, A Comprehensive Examination of Ni@AgCl Nanoparticles – An Insight for Opto-Electronic Applications. J.Con.Ma. 2023;1(2):156-159. Available From https://jcm.thecmrs.in/index.php/j/article/view/29/ |


 ©
© 
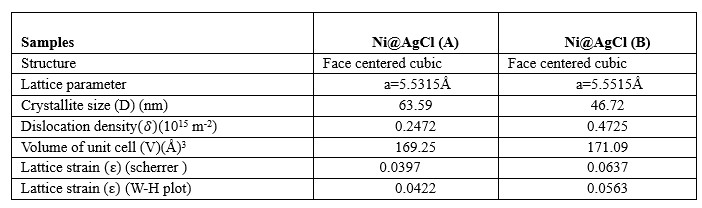
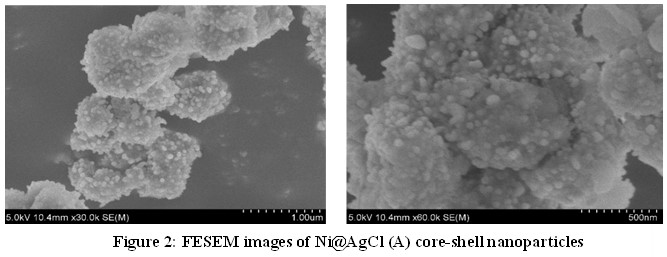 FESEM Analysis
FESEM Analysis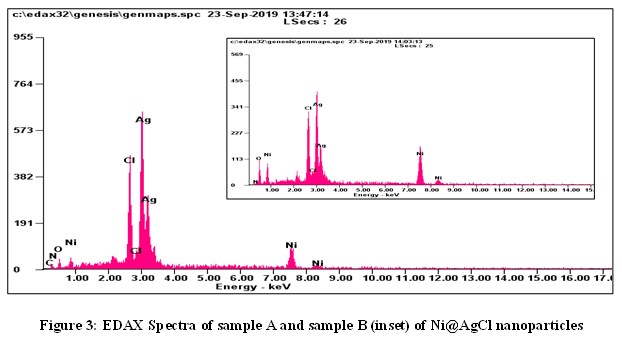 Figure 3 shows EDAX spectra of the synthesized Ni@AgCl core shell nanoparticles of molar ratio 1:1 and 4:1. The elemental composition of the Ni@AgCl core-shell nanoparticles were confirmed by EDAX results. In this spectrum, it is seen that the major elements Ni and AgCl were present. Also, it is identified that the percentage of AgCl is higher than that of Ni element.
Figure 3 shows EDAX spectra of the synthesized Ni@AgCl core shell nanoparticles of molar ratio 1:1 and 4:1. The elemental composition of the Ni@AgCl core-shell nanoparticles were confirmed by EDAX results. In this spectrum, it is seen that the major elements Ni and AgCl were present. Also, it is identified that the percentage of AgCl is higher than that of Ni element.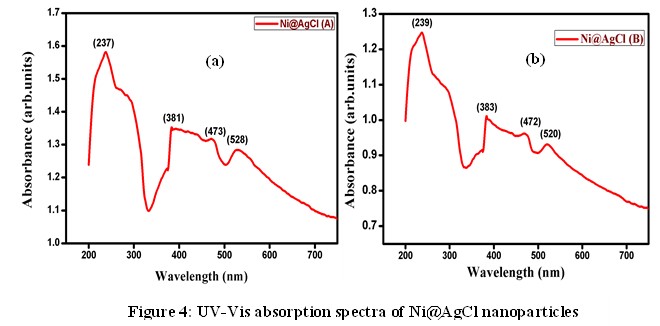 The UV-Vis absorption spectra of the synthesized Ni@AgCl core-shell nanoparticles of molar ratio 1:1 and 4:1 are reported in figure 4. The maximum absorption peak of the sample Ni@AgCl (A) was observed at 237 nm. Also, it gets three additional peaks at 381 nm, 473nm, and 528 nm. Reportedly, the highest absorption peak of 237 nm and 381 nm was assigned to AgCl.
The UV-Vis absorption spectra of the synthesized Ni@AgCl core-shell nanoparticles of molar ratio 1:1 and 4:1 are reported in figure 4. The maximum absorption peak of the sample Ni@AgCl (A) was observed at 237 nm. Also, it gets three additional peaks at 381 nm, 473nm, and 528 nm. Reportedly, the highest absorption peak of 237 nm and 381 nm was assigned to AgCl.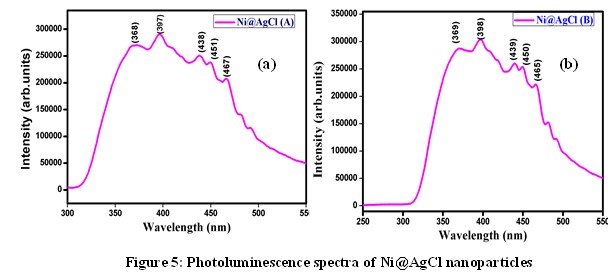 From the PL spectra of the samples A and B, it is clear that the emission of photons is observed at UV region [13]. The sub bands that are responsible for the surface states, play key role in detecting oxygen vacancies and defects on the surface. The synthesized samples Ni@AgCl (A) and Ni@AgCl (B) acting as a semiconducting material and emitting photons at UV region can be used for acoustic devices and optoelectronic applications [14].
From the PL spectra of the samples A and B, it is clear that the emission of photons is observed at UV region [13]. The sub bands that are responsible for the surface states, play key role in detecting oxygen vacancies and defects on the surface. The synthesized samples Ni@AgCl (A) and Ni@AgCl (B) acting as a semiconducting material and emitting photons at UV region can be used for acoustic devices and optoelectronic applications [14].