Needle flower-like ZnO-based chemiresistive sensor for efficient detection of formaldehyde vapors
Mahata B1, Giri S2, Guha P3*
DOI:10.61343/jcm.v1i02.26
1 Bidesh Mahata, School of Nano Science and Technology, Indian Institute of Technology Kharagpur, Kharagpur – 721302, West Bengal, India.
2 Soumen Giri, Materials Science Centre, Indian Institute of Technology Kharagpur, Kharagpur – 721302, West Bengal, India.
3* Prasanta Kumar Guha, Electronics Electrical Communication Engineering, Indian Institute Of Technology Kharagpur, Kharagpur 721302, West Bengal, India.
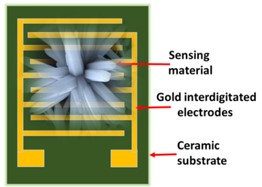
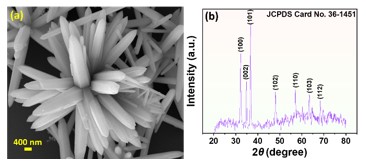
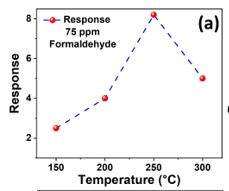
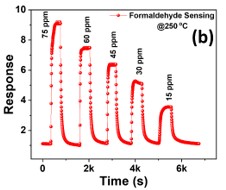
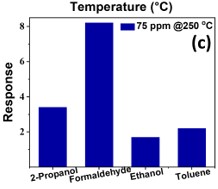
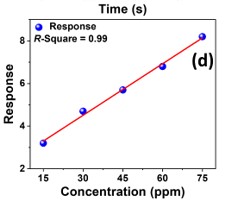
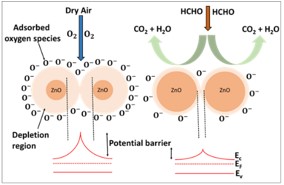
The development of a chemiresistive sensor that uses needle-flower-like ZnO to effectively detect formaldehyde vapors is highlighted in the paper. The hydrothermal process at low temperature was used to prepare the sensing material. The morphological and structural characteristics of the synthesized material were assessed using X-ray diffraction (XRD) and field emission scanning electron microscopy (FESEM). Using a micropipette, the sensing material was transferred to the surface of the gold-based interdigitated electrodes to fabricate the device. The fabricated sensor was found to be more selective and sensitive to formaldehyde in the sensing study. The results showed an approximate response of 8 at 250 °C and 75 ppm formaldehyde. The lowest detection limit of the sensor was calculated as 480 ppb. The sensor has a great potential to monitor formaldehyde vapors in the indoor environment.
Keywords: ZnO, needle flower, chemiresistive, gas sensor, formaldehyde.
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , , Electronics Electrical Communication Engineering, Indian Institute Of Technology Kharagpur, Kharagpur 721302, West Bengal, India. Email: |
Mahata B, Giri S, Guha P, Needle flower-like ZnO-based chemiresistive sensor for efficient detection of formaldehyde vapors. J.Con.Ma. 2023;1(2):123-127. Available From https://jcm.thecmrs.in/index.php/j/article/view/26 |


 ©
©