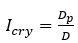Preparation and effect of additives n-ZnO doped p-NiO Screen printed thick films on Structural and Electrical Properties
Mhaske U1*
DOI:10.61343/jcm.v1i02.25
1* Ujwala G Mhaske, Department Of Physics, Hpt Arts And Ryk Science College, Nashik, India.
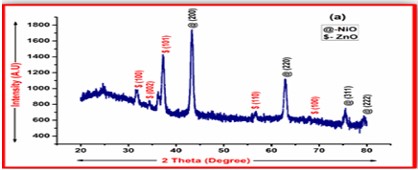
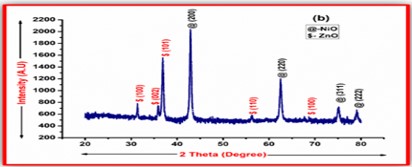
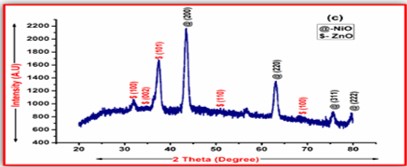
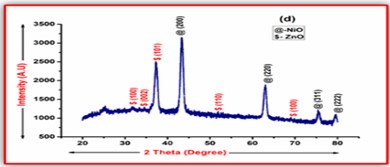
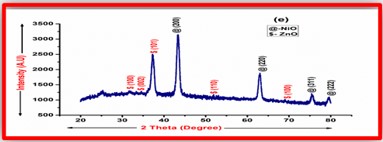
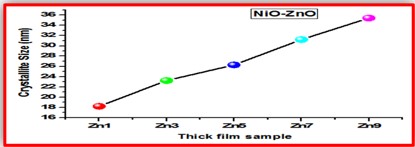
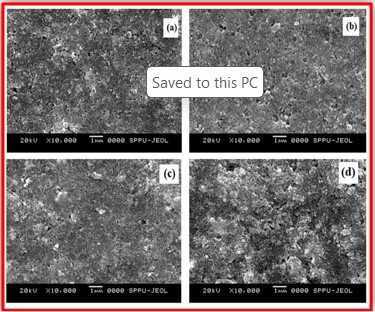
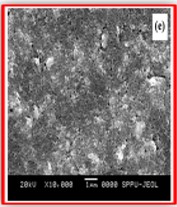
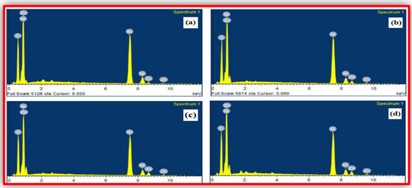
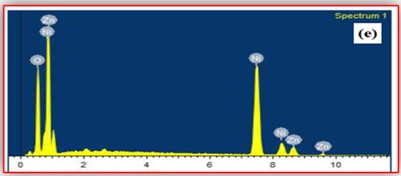
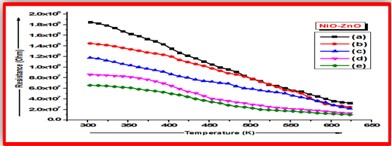
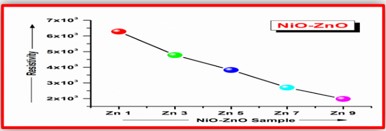
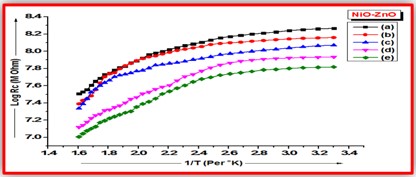
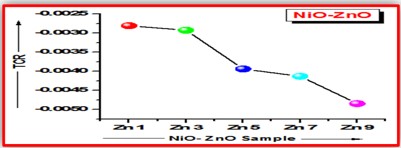
ZnO doped NiO thick films preparation was effectively done using screen printing technique. Using characterisation techniques namely X-ray diffraction (XRD), scanning electron microscopy (SEM) & static gas sensing system, nanoparticles are synthesized. The structural properties of thick films were studied by XRD analysis and its result film shows polycrystalline nature of the films with a cubic structure and crystallite size found to be in the range of 182.1 to 354.4 A.U. SEM analysis of prepared films enabled the conclusion that the prepared films are uniform, large crystals and heavily agglomerated particles were observed spherical in shape. Also, with increase in concentration specific surface area increases. The quantitative chemical compositions were analysed by SEM-EDS and it shows nonstoichiometric in nature. The correlation between structural and morphological properties are reported. Electrical parameters of prepared thick films of ZnO doped NiO nanoparticles namely TCR, activation energy and sheet resistivity, specific surface area were analysed and evaluated at different concentration of zinc oxide. Electrical characterization results resistivity decreases from 6283.38 to 1972.73 Ω-cm with increase in wt.% concentration of ZnO; assured material has a semiconducting in nature. Such a prepared film can be used in fabrication of optoelectronic devices.
Keywords: ZnO, NiO, XRD, SEM-EDS, electrical resistivity, TCR, Activation energy
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , , Department Of Physics, Hpt Arts And Ryk Science College, Nashik, , India. Email: |
Mhaske U, Preparation and effect of additives n-ZnO doped p-NiO Screen printed thick films on Structural and Electrical Properties. J.Con.Ma. 2023;1(2):79-86. Available From https://jcm.thecmrs.in/index.php/j/article/view/25 |


 ©
© 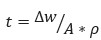 (1)
(1)