A Review on Study of Synthesis, Luminescence Properties and Heating Effect on Cation Doped Garnet Structure
Vandile G1*, Nandanwar D2, Nandanwar A3, Moharil S4
DOI:10.61343/jcm.v1i02.19
1* G C Vandile, Shri M M College Of Science, Nagpur 440009, India.
2 D V Nandanwar, Shri M M College of Science, Nagpur 440009, India.
3 A K Nandanwar, J M Patel Arts Commerce Science College, Bhandara 441904, India.
4 S V Moharil, PGT, Department of Physics, RTMNU, Nagpure 440033, India.
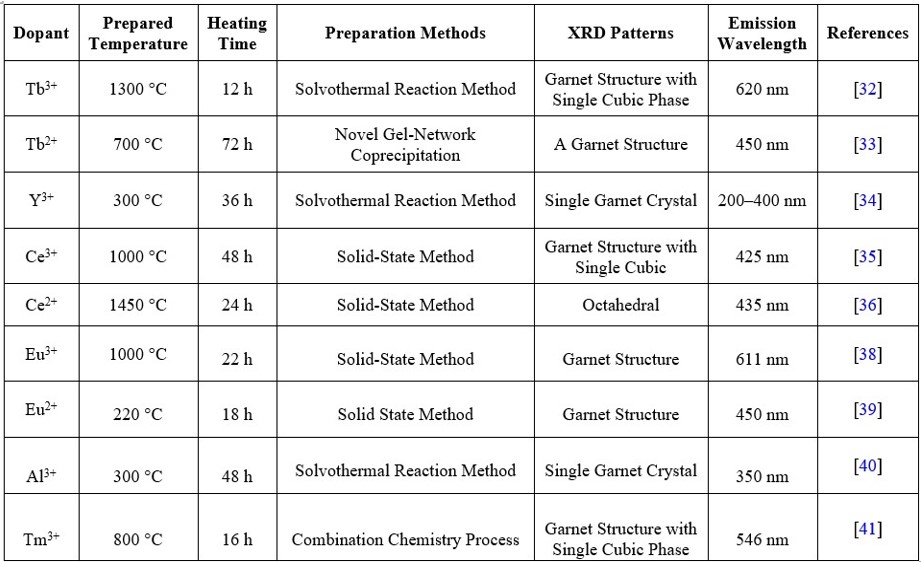
In this review, first we discuss detailed study of garnet structure with various dopant like Tb3+, Tb2+, Y3+, Ce2+, Ce3+, Eu3+, Eu2+, Al3+ and Tm3+ etc. The choice of synthesis method depends on the specific requirements of the application and the desired properties of the garnet-structured nanoparticle phosphors. Researchers often fine-tune the synthesis parameters to achieve the desired particle size, morphology, and luminescent properties. Additionally, post-synthesis treatments, such as annealing or surface modifications, may be employed to further enhance the phosphor's performance. It is observed that the structure of the garnets crystal is cubic and belongs to cubic phase and (Ia3d) space group. The researchers were worked with the garnet components as host and activated by lanthanide trivalent dopants in the range of nanoscale materials. The product materials were used for white LED after investigation of excitation and emission wavelength at different temperature with different heating time.
Keywords: A. Garnet Structure; B. Phosphors; C. Luminescence; D. Heating effect; E. Lanthanide trivalent dopant
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , , , Shri M M College Of Science, Nagpur 440009, , India. Email: |
Vandile G, Nandanwar D, Nandanwar A, Moharil S, A Review on Study of Synthesis, Luminescence Properties and Heating Effect on Cation Doped Garnet Structure. J.Con.Ma. 2023;1(2):69-74. Available From https://jcm.thecmrs.in/index.php/j/article/view/19 |


 ©
©