Study Of Negative Permittivity Behavior Sr7Mn4O15.SrO Nanocomposite
Nirala G1, Verma H2, Baranwal R3, Upadhyay S4*
DOI:10.61343/jcm.v1i02.17
1 Gurudeo Nirala, Department of Physics, Indian Institute of Technology BHU, Varanasi 21005, India.
2 Harish Verma, Department of Physics, Indian Institute of Technology BHU, Varanasi221005, India.
3 Rajni Baranwal, Department of Physics, Indian Institute of Technology BHU, Varanasi 221005, India.
4* Shail Upadhyay, Associate Professor, Department Of Physics, Indian Institute Of Technology Bhu, Varanasi 221005, India.
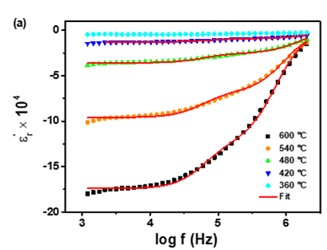
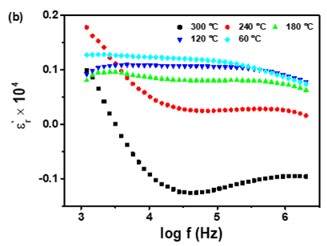
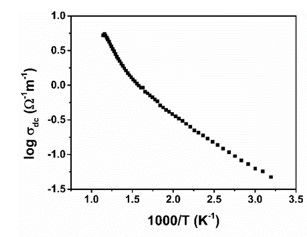
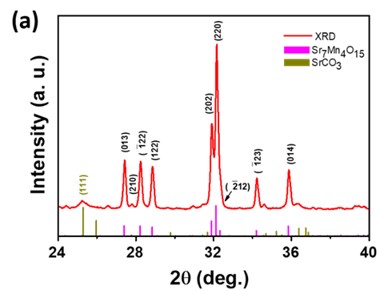
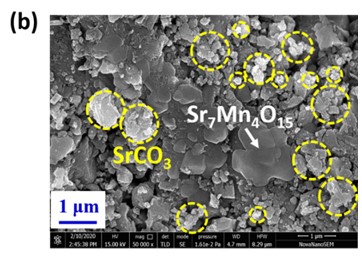
Negative permittivity has been researched extensively in a wide range of metamaterials and composites. Using a solid-state ceramic route, a composite of Sr7Mn4O15 - SrO has been produced. Above a specified temperature (Tc), a change in permittivity sign from positive to negative is observed at all measured frequencies (10 Hz-2MHz). Experimental data of real part of permittivity was fitted to Drude-Lorentz oscillator model. Plasma oscillations of thermally excited free carriers have been identified as the cause of negative permittivity. High temperature plasma plasmonic activity of synthesized composite make it promising metamaterial for electromagnetic devices working in the radio frequency (10 Hz -2MHz) range.
Keywords: Sr7Mn4O15; Composite; Negative permittivity; Drude-Lorentz model
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , Associate Professor, Department Of Physics, Indian Institute Of Technology Bhu, Varanasi 221005, , India. Email: |
Nirala G, Verma H, Baranwal R, Upadhyay S, Study Of Negative Permittivity Behavior Sr7Mn4O15.SrO Nanocomposite. J.Con.Ma. 2023;1(2):166-170. Available From https://jcm.thecmrs.in/index.php/j/article/view/17 |


 ©
© 
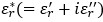 was computed using the following equation from measured impedance,Z* (=Z+iZ'') data:
was computed using the following equation from measured impedance,Z* (=Z+iZ'') data:
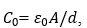 , ε0= free space permittivity, d = separation between electrodes’ surface and A= electrode surface area.
, ε0= free space permittivity, d = separation between electrodes’ surface and A= electrode surface area.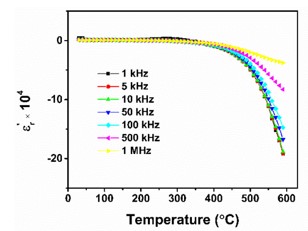
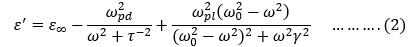
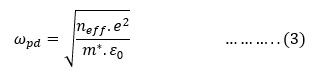 .
.