Plant Based Synthesis of ZnO Nanoparticles and Characterization by UV-Vis Spectroscopy
Bissa S1*, Naruka P2, Birthlya R3, Jain A4
DOI:10.61343/jcm.v1i01.9
1* Shivangi Bissa, Department Of Physics, Govt Engineering College Bikaner, Bikaner, Rajasthan, India.
2 Preeti Naruka, Department of Physics, Govt Engineering College Bikaner, Bikaner, Rajasthan, India.
3 Raj Birthlya, Govt Engineering College Bikaner, Maharaja Ganga Singh University, Bikaner, Rajasthan, India.
4 Arihant Jain, Govt Engineering College Bikaner, Maharaja Ganga Singh University, Bikaner, Rajasthan, India.
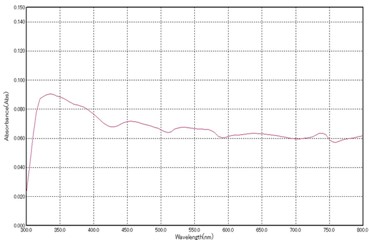
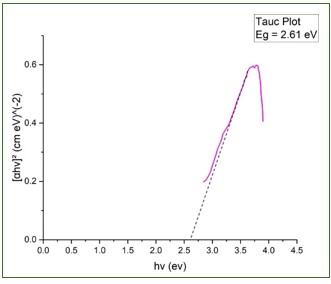
In the modern fast changing world, as the green and environment friendly methods are much required and preferred over conventional toxic methods, plant based nano synthesis has a brilliant scope in future developments of nanotechnology. In the present work, the synthesis and characterization of Zinc Oxide nanoparticles has been discussed by using the green synthesis method which utilizes the leaves of Ocimum Tenuiflorum as reducing agent for Zn salt. ZnO NPs prepared by such a technique exhibit superior antibacterial efficacy against a range of bacteria compared to ZnO NPs produced through chemical methods, without developing resistance to antibiotics. The characterization of ZnO NPs has been done by UV-Vis spectroscopy technique and the Tauc Plot method has been used for calculation of bandgap.
Keywords: Green synthesis, UV-vis Spectroscopy, Ocimum Tenuiflorum, ZnO nanoparticles
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , , Department Of Physics, Govt Engineering College Bikaner, Bikaner, Rajasthan, India. Email: |
Bissa S, Naruka P, Birthlya R, Jain A, Plant Based Synthesis of ZnO Nanoparticles and Characterization by UV-Vis Spectroscopy. J.Con.Ma. 2023;1(1):53-58. Available From https://jcm.thecmrs.in/index.php/j/article/view/9 |


 ©
©