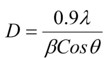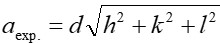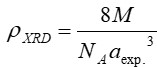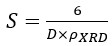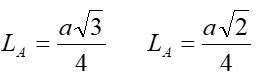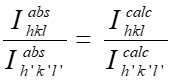Effect of Cation Disorder on Basic Parameters of ZnFe2O4 Nano-Particles Synthesized by Honey Mediated Solution Combustion Technique
Raghuvanshi S1*
DOI:10.61343/jcm.v2i01.39
1* Saroj Raghuvanshi, Shri Cloth Market Institute Of Professional Studies, Indore, Mp, India.
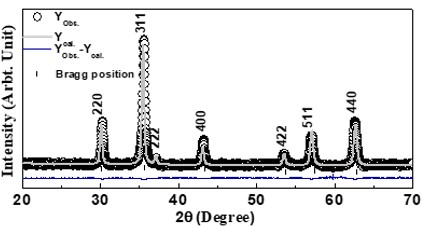
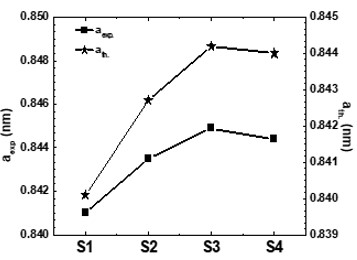
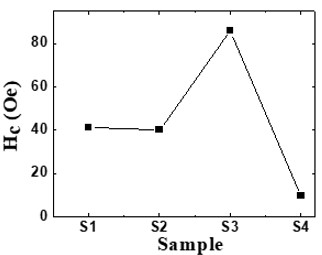
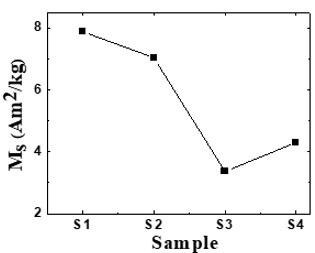
Nanotechnology contracts by the invention and practice of material using nanoscale dimension. Nanoscale measurement delivers nano-particles a bulky superficial area ‘S’ to volume ratio, hence very specific characteristics. Bulk zinc ferrite (ZnFe2O4) exhibits anti-ferromagnetism, with Néel temperature of 10K, is paramagnetic at room temperature. It has a typical spinel structure and exclusive tetrahedral - A site preference when Zn2+ is present, whereas Fe3+ ions occupy the octahedral - B site. Cationic disorder induced fractional overturn of the spinel structure, owing to partial immigration of Fe3+ ions from B to A site can prompt ferrimagnetism in nano zinc ferrite. Owing to the high concentration of hazardous substances and harsh conditions involved in the chemical and physical manufacturing process, a green approach utilizing fungi, bacteria, and plants has been used. Present work reports comprehensive study of the synthesis, structural and magnetic investigation of room temperature ferrimagnetism in ZnFe2O4 nanoparticles, prepared by sol gel auto-combustion mode and green synthesis method. Effect of conventional thermal annealing (ann. at 600oC for 3 hours) on magnetic properties is also reported. The magnetic and structural characteristics of synthesized and annealed ZnFe2O4 samples were determined by vibrating sample magnetometer (VSM) and X-ray diffraction (XRD). XRD verifies that the samples have formed a single-phase nano-crystalline cubic spinel configuration.
Keywords: Zn Ferrite, Green Synthesis, Cationic Disorder
| Corresponding Author | How to Cite this Article | To Browse |
|---|---|---|
| , , , Shri Cloth Market Institute Of Professional Studies, Indore, Mp, India. Email: |
Raghuvanshi S, Effect of Cation Disorder on Basic Parameters of ZnFe2O4 Nano-Particles Synthesized by Honey Mediated Solution Combustion Technique. J.Con.Ma. 2024;2(1):1-5. Available From https://jcm.thecmrs.in/index.php/j/article/view/39 |


 ©
©